Synthesis of tri(di)fluoroethylanilines via copper-catalyzed coupling reaction of tri(di)fluoroethylamine with (hetero)aromatic bromides†
Abstract
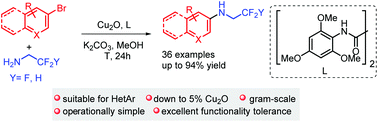
We have realized the first Ullmann type coupling reaction of tri(di)fluoroethylamine with (hetero)aromatic bromides, employing 5–20 mol% Cu2O and an oxalamide ligand [N-(2,4,6-trimethoxyphenyl)acetamide]. This efficient and practical method has the following features: (i) avoids the use of an expensive catalyst; (ii) does not require anhydrous solvent and strict air extrusion; (iii) uses bench stable and inexpensive (hetero)aromatic bromides; (iv) is suitable for the synthesis of fluoroalkylated hetero-aromatic substrates; (v) is suitable for gram-scale synthesis. This work also shows the “negative fluorine effect” for the alkylamines in the copper catalysed coupling reactions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...