Visible-light-induced cascade sulfonylation/cyclization of N-propargylindoles with aryldiazonium tetrafluoroborates via the insertion of sulfur dioxide†
Abstract
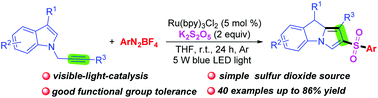
A simple and efficient visible-light-catalyzed cascade sulfonylation/cyclization of N-propargylindoles with K2S2O5 and aryldiazonium tetrafluoroborates for the construction of 2-sulfonyl-substituted 9H-pyrrolo[1,2-a]indoles is developed. In this transformation, K2S2O5 as a simple and inexpensive sulfur dioxide source provides sulfur dioxide for accessing sulfonyl radicals. This method features excellent functional group tolerance and offers a convenient route for assembling a new C–C bond and two new C–S bonds in one step.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...