Copper-catalyzed cascade click/nucleophilic substitution reaction to access fully substituted triazolyl-organosulfurs†
Abstract
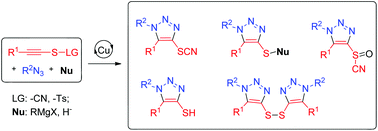
A novel cascade click/nucleophilic substitution reaction is developed to access 4-heterofunctionalized fully substituted triazolyl-organosulfurs using thiocyanates as both leaving groups and organosulfur precursors. This method features high regioselectivities and board substrate scope. 33 examples are shown to demonstrate the structural diversity through the synthesis of fully substituted triazolyl-organosulfurs including triazolyl-thiocyanates, triazolyl-sulfinylcyanides, triazolyl-thioethers, triazolyl-thiols and triazolyl-disulfides from internal thiocyanatoalkynes.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...