Primary amine catalyzed diastereo- and enantioselective Michael reaction of thiazolones and α,β-unsaturated ketones†
Abstract
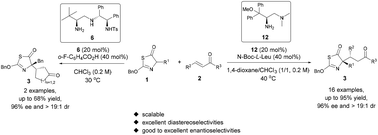
The chiral primary amine catalyzed asymmetric Michael reaction of thiazolones and α,β-unsaturated ketones was reported. Two different optimal catalytic systems were obtained corresponding to cyclic and linear α,β-unsaturated ketones. By employing chiral primary amines as the catalysts and amino-acid derivatives as the additives, a variety of Michael adducts containing the scaffold of the thiazole ring were prepared in moderate to good yields and with excellent diastereo- and enantioselectivities (up to 95% yield, all up to >19/1 dr, up to 96% ee). The reaction was scaled up to obtain 1.73 grams of the Michael adduct with the maintenance of yield and stereoselectivity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...