Cu-Catalysed oxidative amidation of cinnamic acids/arylacetic acids with 2° amines: an efficient synthesis of α-ketoamides†
Abstract
A new and convenient copper-catalysed synthesis of α-ketoamides has been accomplished using readily available cinnamic acids/arylacetic acids and 2° amines in an open atmosphere. The reaction between cinnamic acid and amine involves the formation of enamine followed by its aerobic oxidation, whereas the reaction of arylacetic acid with amine involves amide formation followed by benzylic methylene oxidation.
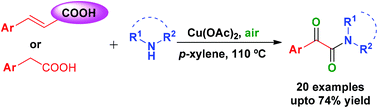


 Please wait while we load your content...
Please wait while we load your content...