Decarboxylative cascade cyclization of α-keto acids with 2-cyano-3-arylaniline-derived acrylamides†
Abstract
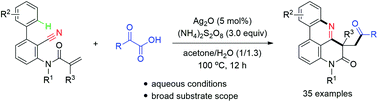
An oxidative decarboxylative cascade cyclization of α-keto acids with 2-cyano-3-arylaniline-derived acrylamides was developed. This cascade reaction exhibits a broad substrate scope, and provides an efficient access to carbonyl-containing pyrido[4,3,2-gh]phenanthridines. A possible mechanism is proposed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...