Enzymatic syntheses of novel carbocyclic scaffolds with a 6,5 + 5,5 ring system by squalene-hopene cyclase†
Abstract
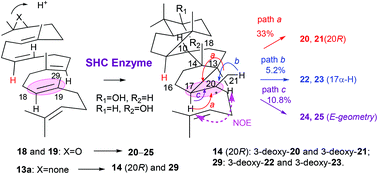
Squalene-hopene cyclase (SHC) converts acyclic squalene 1 into the 6,6,6,6,5-fused pentacyclic triterpenes hopene and hopanol. Previously, we reported the polycyclization products 14–17 of 27-norsqualene (13a) and 28-norsqualene (13b) by SHC, and suggested the importance of Me-27 of 1 for the normal polycyclization pathway. To further ensure the theory, (3R,S)-27-noroxidosqualenes (18 and 19) were incubated, and the structures of products 20–25 thus obtained prompted us to reinvestigate the SHC reaction of 13a (13b). One new product 29, composed of a 6,5 + 5,5 ring system with 13α-H and 17α-H, was obtained from 13a in addition to both the previously isolated products 14–17 and the 6,6,6,5-fused tetracyclic dammarenyl compounds, which were overlooked before. We propose the name “nor-allodammarane” for this novel tetracyclic 6,5 + 5,5 ring system and the name “nor-allogammacerane” for the pentacyclic 6,5 + 5,5 + 6 ring system. The stereochemistry of 29 indicated that 13a folded in the following chair-boat-boat-boat conformation: 10α-H, 11β-H; 14α-H, 15β-Me; 18α-H and 19β-Me, which further allowed us to predict the configuration of 20R for 14 and that of 20S for 15. Substrates 18 and 19 were also cyclized only into allodammarane scaffolds 20–25, and all the structures of 20–25 further indicated that 18 and 19 also folded in the same conformation as 13a, providing further evidence that Me-27 groups of 1 and oxidosqualene are essential for the normal polycyclization pathway by SHC.
- This article is part of the themed collections: Chemical Biology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...