The effect of chiral N-substituents with methyl or trifluoromethyl groups on the catalytic performance of mono- and bifunctional thioureas†
Abstract
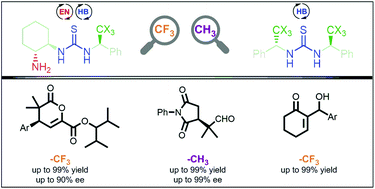
We evaluated thiourea organocatalysts that incorporate a chiral group which includes a trifluoromethyl moiety and contrasted their performance with non-fluorinated analogs. The comparison between such systems allows the direct study of the NH acidity of a thiourea bonded to an aliphatic substituent. In principle, –CF3 systems feature an enhanced hydrogen bond (HB) donor capacity that is undoubtedly beneficial for HB-catalysis applied to the Baylis–Hillman reaction. We found that the thiourea substituted on both nitrogens with this group accelerates this reaction like Schreiner's thiourea. On the other hand, we observed a different behavior in reactions promoted by bifunctional catalysts (thiourea–primary amine). In the Michael addition of isobutyraldehyde to methyl benzylidenepyruvate, the –CF3 containing catalysts were better than the –CH3 systems, whereas the conjugate addition to N-phenylmaleimide showed the opposite behavior. Theoretical calculations of the transition states indicated that the phenylethyl group in fluorinated and non-fluorinated compounds have different kinds of interactions with the electrophile. These interactions are responsible for a different arrangement of the electrophile and thereby the selectivity of the catalyst. Therefore, it cannot be generalized that in all cases NH acidity correlates with the performance of the catalyst, particularly, with aliphatic substituents that unlike the aromatic ones possess groups that are outside the plane of the thiourea.
- This article is part of the themed collection: Celebrating Latin American Talent in Chemistry


 Please wait while we load your content...
Please wait while we load your content...