Origins of stereoselectivity in uncatalyzed and ZnBr2-catalyzed Diels–Alder reactions of a chiral sulfinylquinone†
Abstract
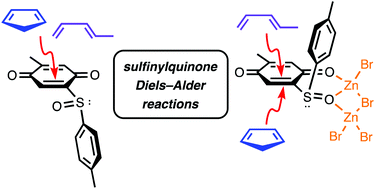
Density functional theory calculations are reported which explore how a chiral sulfinyl substituent controls the stereoselectivities of the Diels–Alder reactions of a 2-p-tolylsulfinylbenzoquinone. The π-facial stereoselectivities vary depending on the diene (cyclopentadiene or trans-piperylene) and on the presence or absence of a ZnBr2 catalyst. The stereoselectivities are shown to be controlled by steric effects and non-covalent (CH–π) interactions. The calculations reveal that the active dienophile in the ZnBr2-catalyzed reactions is likely to be a 1 : 2 complex of the dienophile and catalyst, not a 1 : 1 complex as commonly assumed.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...