Transition metal-free synthesis of quinazolinones using dimethyl sulfoxide as a synthon†
Abstract
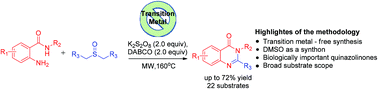
Biologically important quinazolinones have been synthesized from 2-aminobenzamides and DMSO. The key feature of the reaction is the utilization of DMSO as a methine source for intramolecular oxidative annulation. The CNS depressant drug methaqualone has also been synthesized by our methodology. The present method involves the synthesis of quinazolinones with a broad substrate scope and a good yield.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...