Trichloroacetonitrile as an efficient activating agent for the ipso-hydroxylation of arylboronic acids to phenolic compounds†
Abstract
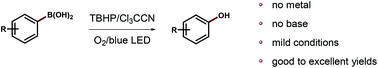
A metal-free and base-free Cl3CCN mediated method was developed for the ipso-hydroxylation of aryl boronic acids to their corresponding phenols, which was promoted by a key unstable Lewis adduct intermediate. This transformation has broad functional group tolerance, and late-stage functionalization was successful as well. After simple investigation, two pathways (radical/ionic mechanism) were suggested, and the beneficial action of blue light needs to be further studied.


 Please wait while we load your content...
Please wait while we load your content...