(Z)-Tetrahydrothiophene and (Z)-tetrahydrothiopyran synthesis through nucleophilic substitution and intramolecular cycloaddition of alkynyl halides and EtOCS2K†
Abstract
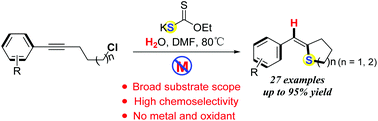
This protocol provides a novel, environmentally friendly and simple method for the synthesis of (Z)-tetrahydrothiophene derivatives using the nucleophilic thiyl radical intramolecular cycloaddition cascade process to construct C–S bonds under transition-metal-free conditions. This transformation process offers a broad substrate scope, good functional group tolerance, and excellent stereoselectivity (Z/E ratios up to 99/1). Moreover, the process uses odourless, stable and cheap EtOCS2K as the sulfur source.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...