Application of isatin-derived saturated esters in the synthesis of 3,3′-spirooxindole γ-butyrolactams†
Abstract
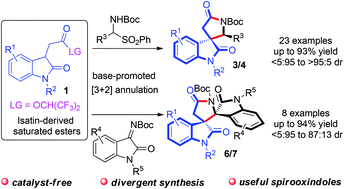
Stable while reactive isatin-derived saturated esters have been utilized as 3-carbon synthons in a base-promoted formal [3 + 2] annulation with N-Boc imines. The developed protocol offers a direct pathway for the rapid and divergent construction of two classes of 3,3′-spirooxindole γ-butyrolactam skeletons that are recognized as the privileged structures of various bioactive compounds. This protocol also has the advantages of mild reaction conditions, scalability and wide reaction scope.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...