NIS-mediated oxidative arene C(sp2)–H amidation toward 3,4-dihydro-2(1H)-quinolinone, phenanthridone, and N-fused spirolactam derivatives†
Abstract
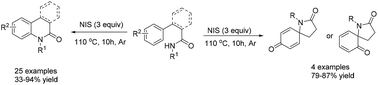
A new radical-mediated intramolecular arene C(sp2)–H amidation of 3-phenylpropanamides or [1,1′-biphenyl]-2-carboxamides was developed to prepare a series of 3,4-dihydro-2(1H)-quinolinone and phenanthridone derivatives in moderate to excellent yields (33–94%). Spirolactams could also be obtained using this protocol.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...