Copper-catalyzed synthesis of spiro-indolofurobenzopyrans: tandem reactions of diazoamides and O-propargyl salicylaldehydes†
Abstract
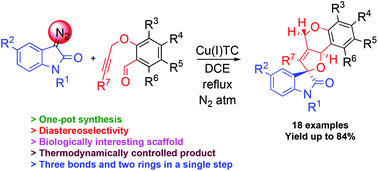
An atom-economical synthesis of spiro-indolofurobenzopyrans was developed from diazoamides and O-propargyl salicylaldehydes in the presence of copper(I) thiophene-2-carboxylate in a diastereoselective manner. This methodology involves the preparation of carbonyl ylide intermediates followed by 1,3-dipolar cycloaddition with internal/external alkynes, offering a great potential for constructing biologically significant spiro-indolofurobenzopyrans, as thermodynamically controlled products, in a tandem manner.


 Please wait while we load your content...
Please wait while we load your content...