Transition-metal free oxidative C–H etherification of acylanilines with alcohols through a radical pathway†
Abstract
A transition metal free approach for the synthesis of methyl/ethyl aryl ether via oxidative C–H etherification of acylanilines with alcohols has been developed. Various acylanilines are compatible under standard conditions, giving the corresponding products in moderate to good yields. This strategy avoids transition-metal catalyst and excessive alcohol, providing a simple and reliable alternative method for the synthesis of methyl/ethyl aryl ether. Control experiments reveal that a radical mechanism is involved in this transformation.
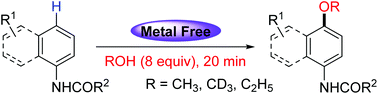
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...