Synergistic palladium/enamine catalysis for asymmetric hydrocarbon functionalization of unactivated alkenes with ketones†
Abstract
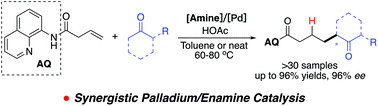
Synergistic palladium and enamine catalysis was explored to promote ketone addition to unactivated olefins. A secondary amine-based organocatalyst was identified as the optimal co-catalyst for the directed Pd-catalyzed alkene activation. Furthermore, asymmetric hydrocarbon functionalization of unactivated alkenes was also achieved with good to excellent yield (up to 96% yields) and stereoselectivity (up to 96% ee). This strategy presented an efficient approach to prepare α-branched ketone derivatives under mild conditions.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...