An organocatalytic method for constructing pyrroles via the cycloisomerisation of Z-1-iodo-4-N-methylbenzenesulfonyl-1,6-enynes†
Abstract
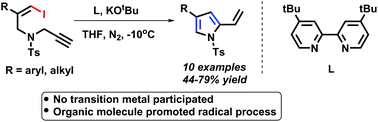
A new cycloisomerisation of Z-1-iodo-4-N-methylbenzenesulfonyl-1,6-enynes to functionalized pyrroles was realized in the presence of an organomolecule (4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine) and KOtBu. The transformations were performed efficiently to produce different kinds of functionalized pyrroles within 10 min. This is the first example of an organomolecule promoted methodology with vinyl iodides from a non-aromatic system to an aromatic system, which offers an excellent option toward establishing a new horizon for cross-coupling reactions of vinyl halides. Preliminary mechanistic studies were performed and a crude radical pathway was proposed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...