Catalytic asymmetric (4 + 1) annulation of nitroalkenes with allylic acetates: stereoselective synthesis of isoxazoline N-oxides†‡
Abstract
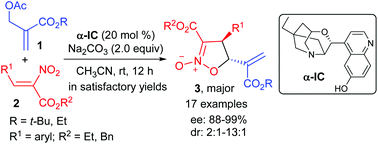
An asymmetric (4 + 1) annulation of α-nitro cinnamates with Morita–Baylis–Hillman (MBH) acetates catalyzed by α-isocupreine is reported. It provides chiral isoxazoline N-oxides in moderate to good yields with 88–99% ee, and represents the first catalytic asymmetric (4 + 1) annulation of activated nitroalkenes with in situ generated ammonium ylides. It also affords a practical and efficient access to chiral isoxazoline N-oxides.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...