Thermal rearrangement of optically active tetradeuterated 2-methoxymethyl-methylenecyclopropane and the bent bond/antiperiplanar hypothesis†
Abstract
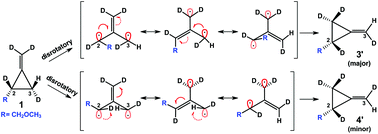
The thermolysis of an optically active tetradeuterated 2-methoxymethyl methylenecyclopropane produces a specific ratio of eight possible rearrangement stereoisomers. Despite numerous efforts, this reaction and other similar transformations have defied mechanistic interpretation until now. The direct application of the bent bond/antiperiplanar hypothesis (BBAH) to this reaction produces a mechanistic model that rationalizes all the observed reaction kinetics and products. The BBAH dictates that allyl diradical intermediates, produced during methylenecyclopropane thermolysis, retain pyramidal character due to antiperiplanar delocalization into their respective bent bond.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...