Employment of α-nitroketones in organic synthesis
Abstract
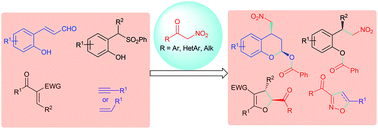
α-Nitroketones, useful bidentate reagents having nitro and keto functionalities, have been recently employed in a range of metal and organocatalytic domino reactions. In fact, several C–C, C–O, and C–N bond forming reactions were developed including Michael, domino-Michael, denitration, desymmetrisation, Mannich, nucleophilic addition/elimination and cycloaddition pathways. Unlike nitroalkanes, α-nitroketones can generate a range of active 1,3 dipolar intermediates such as nitronates and nitrile oxides which have been engaged in various cycloaddition reactions. Also α-functionalisation reactions such as arylations and halogenations were carried out. A variety of useful organic frameworks such as chromans, dihydrofurans, isoxazoles, pyrazoles etc. have been prepared. This review highlights the recent developments in the utilisation of α-nitroketones in organic synthesis and discusses the progress over the current years.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...