Regioselective oxidation of indoles to 2-oxindoles†
Abstract
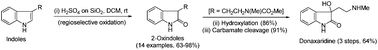
Facile regioselective oxidation of indoles to 2-oxindoles promoted by sulfuric acid adsorbed on silica gel is reported. The demonstrated practical site-selective heterogeneous oxidation reactions conveniently take place with a broad substrate scope and functional group tolerances. The present oxidation strategy is also employed to accomplish the total synthesis of natural products donaxaridine and donaxarine. On the basis of analytical and spectral data it is evidenced that donaxarine stays in equilibrium with its hydrated ring opened form. The structural features essential for this type of oxidation and plausible mechanism are discussed in brief.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...