Synthesis and in vitro evaluation of fluorine-18 benzimidazole sulfones as CB2 PET-radioligands†
Abstract
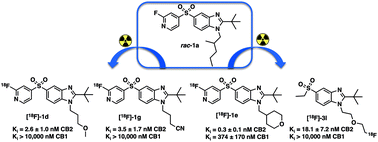
Cannabinoid type 2 receptor (CB2) is up-regulated on activated microglial cells and can potentially be used as a biomarker for PET-imaging of neuroinflammation. In this study the synthesis and pharmacological evaluation of novel fluorinated pyridyl and ethyl sulfone analogues of 2-(tert-butyl)-5-((2-fluoropyridin-4-yl)sulfonyl)-1-(2-methylpentyl)-1H-benzo[d]imidazole (rac-1a) are described. In general, the ligands showed low nanomolar potency (CB2 EC50 < 10 nM) and excellent selectivity over the CB1 subtype (>10 000×). Selected ligands 1d, 1e, 1g and 3l showing high CB2 binding affinity (Ki < 10 nM) were radiolabelled with fluorine-18 from chloropyridyl and alkyl tosylate precursors with good to high isolated radioactive yields (25–44%, non-decay corrected, at the end of synthesis). CB2-specific binding of the radioligand candidates [18F]-1d and [18F]-3l was assessed on rat spleen cryosections using in vitro autoradiography. The results warrant further in vivo evaluation of the tracer candidates as prospective CB2 PET-imaging agents.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...