Synthesis and antiproliferative evaluation of new zampanolide mimics†
Abstract
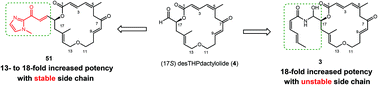
(−)-Zampanolide is a marine microtubule-stabilizing macrolide that has been shown by in vitro experiments to be a promising anticancer lead compound. Through its unique covalent-binding with β-tubulin, zampanolide exhibits cytotoxic potency towards multi-drug resistant cancer cells that is superior to paclitaxel. However, the limited availability of zampanolide impedes its further in vivo evaluation as a viable drug candidate. Zampanolide is envisioned to become more drug-like if its chemically fragile side chain can be stabilized; hence, this project aims to develop mimics of zampanolide with a stable side chain using straightforward synthetic methods. To this end, twelve novel zampanolide mimics (51–62) with conjugated and planar side chains have been synthesized via a 24-step sequence for each mimic from commercially available 2-butyn-1-ol as starting material. A Horner–Wadsworth–Emmons reaction incorporates the α,β-unsaturated ketone side chain and also closes the core macrocycle. WST-1 cell proliferation assays in three docetaxel-sensitive and two docetaxel-resistant human prostate cancer cell models confirm that a suitably designed side chain can serve as a bioisostere for the N-acyl hemiaminal side chain in zampanolide. Mimic 52 with a 17R chiral center was identified as the optimal candidate with IC50 values of 0.29–0.46 μM against both docetaxel-sensitive (PC-3 and DU145) and docetaxel-resistant prostate cancer cell lines (PC-3/DTX and DU145/DTX). Zampanolide mimic 52 exhibited equivalent antiproliferative potency towards both docetaxel-sensitive and docetaxel-resistant cell lines, with relative resistance in the range of 0.9–1.6.


 Please wait while we load your content...
Please wait while we load your content...