TuPPL: Tub-tag mediated C-terminal protein–protein-ligation using complementary click-chemistry handles†
Abstract
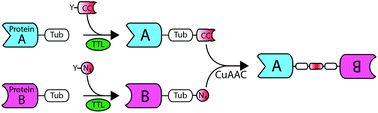
We introduce a chemoenzymatic strategy for straightforward in vitro generation of C-terminally linked fusion proteins. Tubulin tyrosine ligase is used for the incorporation of complementary click chemistry handles facilitating subsequent formation of functional bispecific antibody-fragments. This simple strategy may serve as central conjugation hub for a modular protein ligation platform.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...