On the reactivity of anodically generated trifluoromethyl radicals toward aryl alkynes in organic/aqueous media†
Abstract
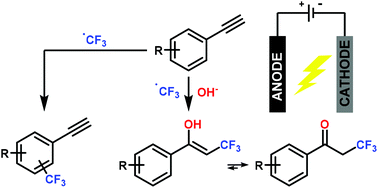
An in-depth study of the reaction of electrochemically generated trifluoromethyl radicals with aryl alkynes in the presence of water is presented. The radicals are readily generated by anodic oxidation of sodium triflinate, an inexpensive and readily available CF3 source, with concomitant reduction of water. Two competitive pathways, i.e. aryl trifluoromethylation vs. oxytrifluoromethylation of the alkyne, which ultimately lead to the generation of α-trifluoromethyl ketones, have been observed. The influence of several reaction parameters on the reaction selectivity, including solvent effects, electrode materials and substitution patterns on the aromatic ring of the substrate, has been investigated. A mechanistic rationale for the generation α-trifluoromethyl ketones based on cyclic voltammetry data and radical trapping experiments is also presented. DFT calculations carried out at the M06-2X/6-311+G(d,p) level on the two competing pathways account for the observed selectivity.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...