Synthesis and characterization of propeller-shaped mono- to hexacationic quinolinium-substituted benzenes†
Abstract
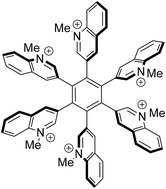
Diels–Alder reaction of 2-, 3- and 4-(phenylethynyl)quinolines and tetraphenylcyclopentadienone gave three regioisomeric 2,3,4,5,6-pentaphenyl-1-(quinolin-2-yl, -3-yl, and -4-yl)benzenes. Restricted rotation of the 3-yl and 4-yl substituted derivatives is observed between the central core and the substituents, resulting in propeller-shaped molecules. Likewise, 1,2-diquinolinyl-3,4,5,6-tetraphenylbenzenes with 3-yl,3-yl and 3-yl,4-yl connectivity were prepared. As evidenced by NMR spectroscopy, they form two diasteromers due to their restricted rotation. A cobalt-catalyzed [2 + 2 + 2]-cyclotrimerization of 2-(phenylethynyl)quinoline resulted in the formation of triphenyl-2,4,6- and -3,5,6-tri(quinolin-2-yl)benzenes. The same reaction was applied to 3,3′-ethyne-1,2-diyldiquinoline which formed hexa(quinolin-3-yl)benzene. N-Methylation gave the title compounds. Among those, the hexacationic hexa(N-methylquinolinio-3-yl)benzene is described. Stereochemical aspects are predominantly discussed by means of results of NMR experiments. DFT-calculations on the most stable conformations and the frontier orbital profiles of the hexacation as well as of its neutral precursor have been carried out.
- This article is part of the themed collections: Synthetic methodology in OBC and Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...