Visible-light-induced radical trifluoromethylthiolation of N-(o-cyanobiaryl)acrylamides†
Abstract
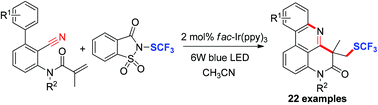
An efficient and general visible-light-mediated trifluoromethylthiolation of N-(o-cyanobiaryl)acrylamides has been successfully accomplished using N-trifluoromethylthiosaccharin as an effective source of SCF3 radicals. The reaction was proposed to proceed via a domino radical trifluoromethylthiolation/cyano insertion/cyclization to afford the corresponding SCF3-containing ring-fused phenanthridine derivatives in moderate to good yields.
- This article is part of the themed collections: Synthetic methodology in OBC and Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...