N-Hydroxyphthalimide/benzoquinone-catalyzed chlorination of hydrocarbon C–H bond using N-chlorosuccinimide†
Abstract
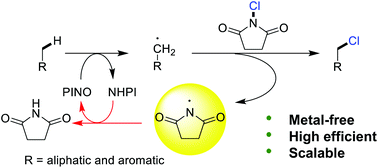
The direct chlorination of C–H bonds has received considerable attention in recent years. In this work, a metal-free protocol for hydrocarbon C–H bond chlorination with commercially available N-chlorosuccinimide (NCS) catalyzed by N-hydroxyphthalimide (NHPI) with 2,3-dicyano-5,6-dichlorobenzoquinone (DDQ) functioning as an external radical initiator is presented. Aliphatic and benzylic substituents and also heteroaromatic ones were found to be well tolerated. Both the experiments and theoretical analysis indicate that the reaction goes through a process wherein NHPI functions as a catalyst rather than as an initiator. On the other hand, the hydrogen abstraction of the C–H bond conducted by a PINO species rather than the highly reactive N-centered radicals rationalizes the high chemoselectivity of the monochlorination obtained by this protocol as the latter is reactive towards the C(sp3)–H bonds of the monochlorides. The present results could hold promise for further development of a nitroxy-radical system for the highly selective functionalization of the aliphatic and benzylic hydrocarbon C–H.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...