Highly efficient regio-selective ring-opening nucleophilic fluorination of aziridines and azetidines: access to β- or γ-fluorinated amino acid derivatives†
Abstract
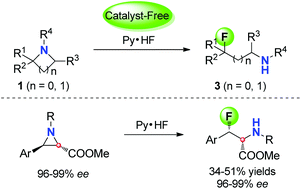
Herein, we have presented a facile and efficient method of ring-opening nucleophilic fluorination of aziridines, affording highly regio-selective β-fluorinated amines. Firstly, the example of ring-opening hydrofluorination of azetidines was reported. Then, the Olah's reagent also provided a promising method for the construction of enantioenriched β-fluoro-α-amino acid derivatives, which could be used for the preparation of peptide-based bioactive molecules.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...