Zn(OTf)2-catalyzed access to symmetrical and unsymmetrical bisindoles from α-keto amides†
Abstract
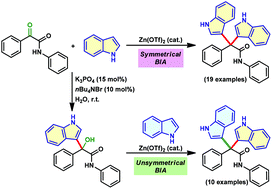
Zn(OTf)2-catalyzed synthesis of 3,3′-bisindolyl acetamides from α-keto amides is developed. Both aromatic α-keto amides substituted with electron-donating as well as -withdrawing groups and aliphatic α-keto amides are well tolerated to provide symmetrical bisindoles in moderate to excellent yields. The chemoselective bisindolylation of the keto group of α-keto amides in the presence of a simple keto functionality is successfully achieved in good yields. The transformation is further extended to the synthesis of challenging unsymmetrical bisindoles by treating indolyl α-hydroxy amides with substituted indoles. The unsymmetrical bisindoles are isolated in good to excellent yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...