Rhodium-catalyzed direct C–H bond alkynylation of aryl sulfonamides with bromoalkynes†
Abstract
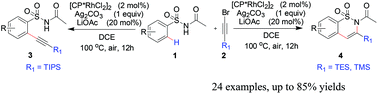
Herein we report a novel rhodium-catalyzed ortho-mono-alkynylation of aryl sulfonamides. The reactions of N-tosylacetamides with triisopropylsilyl (TIPS)-substituted bromoalkyne are catalyzed by a [(Cp*RhCl2)2] complex without cyclization, forming ortho-(1-alkynyl) benzenesulfonamides. While triethylsilyl or trimethylsilyl (TES or TMS)-substituted bromoalkyne was also amenable to the alkynylation, affording six-membered benzosultams via the alkynylation/intramolecular cyclization cascade reaction. The present protocol displays high functional group tolerance and broad substrate scope under an air atmosphere in good to high yields. Mechanistic studies indicate that the reaction proceeds by a turnover limiting C–H activation step and a plausible mechanism was proposed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...