Towards 20,20-difluorinated bryostatin: synthesis and biological evaluation of C17,C27-fragments†
Abstract
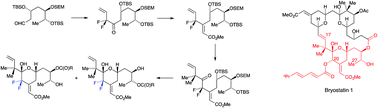
Bryostatins with modified C17–C27 fragments have not been widely studied. The synthesis of 20,20-difluorinated analogues was therefore investigated. Such substitution would inhibit dehydration involving the C19-hydroxyl group and stabilise the ring-closed hemiacetal tautomers. Following preliminary studies, allyldifluorination was used to prepare difluorinated alkenols. Oxidation followed by stereoselective Wittig reactions of the resulting α,α-difluorinated ketones gave (E)-α,β-unsaturated esters that were taken through to complete syntheses of 2-hydroxytetrahydropyrans corresponding to C17–C27 fragments of 20,20-difluorinated bryostatin. These compounds showed modest binding to protein kinase Cα isozyme. Attempts were also undertaken to synthesise macrocyclic 20,20-difluorinated analogues. During preliminary studies, allyldifluorination was carried out using a 2-alkyl-3-bromo-1,1-difluoropropene.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...