One-pot synthesis of 4-arylidene imidazolin-5-ones by reaction of amino acid esters with isocyanates and α-bromoketones†
Abstract
A simple and new multicomponent reaction for the one-pot synthesis of substituted 4-arylidene imidazolin-5-ones from L-amino acid methyl esters, iso-, isothio- or isoselenocyanates, and α-bromoketones is demonstrated. Isolation of thiohydantoin and 5-benzylidene 2-thioxoimidazolidin-4-one intermediates revealed a possible reaction mechanism. The strategy was further extended to the synthesis of 2-iminothiazolines and 2-thioxoimidazolin-4-ones.
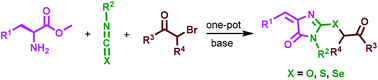


 Please wait while we load your content...
Please wait while we load your content...