Absolute asymmetric Strecker synthesis in a mixed aqueous medium: reliable access to enantioenriched α-aminonitrile†
Abstract
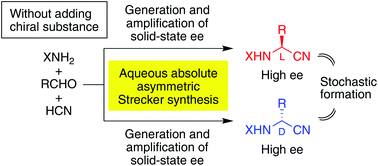
Without using chiral sources, the Strecker reaction of achiral hydrogen cyanide, p-tolualdehyde and benzhydrylamine gave enantioenriched L- or D-N-benzhydryl-α-(p-tolyl)glycine nitriles with up to >99% ee in a mixed solvent of water and methanol. Therefore, total spontaneous resolution of α-aminonitriles could occur through a prebiotic mechanism of α-amino acid synthesis. Moreover, it was demonstrated that the repetition of partial dissolution and crystallization of a suspended conglomerate of aminonitrile under solution-phase racemization could generate the enantiomeric imbalance to afford, in combination with the amplification of chirality, an enantioenriched product in every case. Among the 73 experiments that were carried out, D- and L-enriched isomers occurred 36 and 37 times, respectively. This stochastic behavior, under achiral or racemic starting conditions, meets the requirements of the spontaneous absolute asymmetric Strecker synthesis. The implications of the present results for the origin of chirality of α-amino acids are discussed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...