Fluorinated bisbenzimidazoles: a new class of drug-like anion transporters with chloride-mediated, cell apoptosis-inducing activity†
Abstract
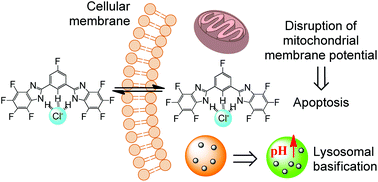
Anion transporters have attracted substantial interest due to their ability to induce cell apoptosis by disrupting cellular anion homeostasis. In this paper we describe the synthesis, anion recognition, transmembrane anion transport and cell apoptosis-inducing activity of a series of fluorinated 1,3-bis(benzimidazol-2-yl)benzene derivatives. These compounds were synthesized from the condensation of 1,3-benzenedialdehyde or 5-fluoro-1,3-benzenedialdehyde with the corresponding 1,2-benzenediamines and fully characterized. They are able to form stable complexes with chloride anions, and exhibit potent liposomal and in vitro anionophoric activity. Their anion transport efficiency may be ameliorated by the total number of fluorine atoms, and the enhanced anionophoric activity was a likely consequence of the increased lipophilicity induced by fluorination. Most of these fluorinated bisbenzimidazoles exhibit potent cytotoxicity toward the selected cancer cells. Mechanistic investigations suggest that these compounds are able to trigger cell apoptosis probably by disrupting the homeostasis of chloride anions.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...