Enantiomeric NMR discrimination of carboxylic acids using actinomycin D as a chiral solvating agent†
Abstract
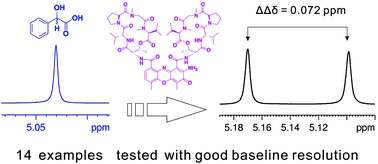
Actinomycin D (Act-D) is a biologically important polypeptide antibiotic clinically used to treat several malignant tumors. Herein, we extended its hitherto-unexplored application as an applicable chiral solvating agent (CSA) for the rapid enantiomeric determination of different chiral carboxylic acids in deuterated chloroform by 1H NMR spectroscopy. Notable enantiodiscrimination with well-splitting α-H or α-CH3 resonance signals of the enantiomers of carboxylic acids were achieved without significant interference from Act-D. To check its applicability for the determination of enantiomeric excess (ee) values, various mandelic acid (MA) samples were determined and compared with the observed ones, resulting in an excellent linear relationship. To our knowledge, this is the first example of using a natural antibiotic compound as a CSA to achieve chiral recognition for carboxylic acids.


 Please wait while we load your content...
Please wait while we load your content...