Chemistry of detrifluoroacetylatively in situ generated fluoro-enolates
Abstract
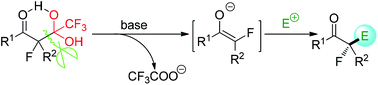
This review article comprehensively profiles all literature reports (2015–2018) related to the detrifluoroacetylative in situ generation of fluorine-containing enolates and their reactions with electrophilic reagents. The innovative facets of this unconventional methodology and its synthetic generality for the preparation of fluorine-containing compounds of high medicinal value are highlighted.
- This article is part of the themed collections: Synthetic methodology in OBC and Fluorine Chemistry


 Please wait while we load your content...
Please wait while we load your content...