Synthesis of (2-hydroxyphenyl)(fusedphenyl)methanones via the photo-induced rearrangement of 2′-arylisoflavones†
Abstract
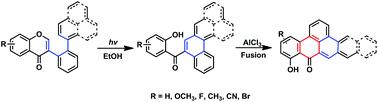
The photo-induced rearrangement of 2′-arylisoflavones (aryl = phenyl, furyl, 3-pyridyl, 1-naphenyl, and 2-naphenyl) for the synthesis of (2-hydroxyphenyl)(fused phenyl)methanones in EtOH under an Ar atmosphere at room temperature has been developed. The described method proceeded smoothly without requiring any transition metal catalyst, oxidant, or additives. Moreover, using ethanol as the solvent is not only cost efficient but also environmentally friendly. Further treatment of methanone analogues with AlCl3 yielded highly conjugated polycyclic aromatic hydrocarbon (PAH) derivatives.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...