Engineering the coupling interface of rhombic dodecahedral NiCoP/C@FeOOH nanocages toward enhanced water oxidation†
Abstract
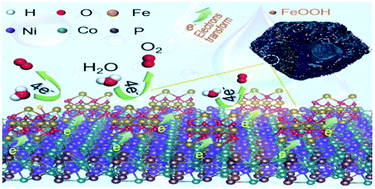
Hydrogen, regarded as one of the most promising green and sustainable energy resources, could be generated by splitting water with electrochemical methods. The challenge for efficient hydrogen generation is the sluggish kinetics at the anodes for the oxygen evolution reaction (OER). Here, a novel catalyst with remarkably enhanced OER activity was prepared by coupling FeOOH and NiCoP/C. The enhanced OER activity of the hybrid catalyst should be ascribed to the synergistic effect of the individual components. First, NiCoP/C derived from ZIF-67 with a hollow rhombic dodecahedral architecture not only allows exposure of numerous active sites but also provides high conductivity. Second, the re-localization of electrons at the coupling interface optimizes the adsorption/desorption nature of intermediate oxygenated species and imparts a high OER activity. The hybrid NiCoP/C@FeOOH catalyst exhibits very high OER activity with a low overpotential of 271 mV for producing a current density of 10 mA cm−2 in 1 M KOH aqueous solution, markedly surpassing the individual counterparts of pure NiCoP/C nanocages and bare FeOOH. This work represents a universal strategy for boosting the OER kinetics of catalysts and pushing boundaries for high-efficiency water oxidation.


 Please wait while we load your content...
Please wait while we load your content...