Plasma-modified Ti3C2Tx/CdS hybrids with oxygen-containing groups for high-efficiency photocatalytic hydrogen production†
Abstract
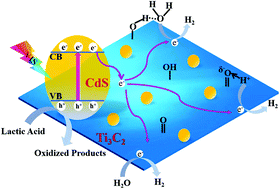
Noble-metal-free Ti3C2Tx/CdS hybrids with oxygen-containing groups were successfully prepared through a mild solvothermal method of in situ heterogeneous nucleation. The abundant oxygen-containing groups and the increased surface roughness of plasma-treated Ti3C2Tx sheets provided active sites for the heterogeneous nucleation of CdS nanoparticles and strengthened the physical bond between CdS NPs and layered Ti3C2Tx. The optimized Ti3C2Tx/CdS hybrid without noble-metal co-catalyst achieved a high hydrogen-production rate of 825 μmol h−1 g−1 with an apparent quantum efficiency of 10.2% at 450 nm. The prepared Ti3C2Tx/CdS hybrid with 1.0 wt% Ti3C2Tx had a photocatalytic hydrogen-production rate higher than those of pure CdS nanoparticles and the Ti3C2/CdS hybrid obtained using non-plasma-treated Ti3C2 as the support matrix under visible-light irradiation. The photocatalytic-activity improvement of CdS nanoparticles was due to the increased hydrophilicity after plasma treatment with the abundant oxygen-containing groups on the Ti3C2Tx surface and the intimate contact between CdS nanoparticles and layered Ti3C2Tx. The former enabled the effective capture of water molecules and hydrogen ions by oxygen-containing groups on the surface of plasma-treated Ti3C2Tx. The latter provided a stable transfer channel for electrons to suppress the recombination of photogenerated electron–hole pairs. The proposed schematic for the improved photocatalytic activity of CdS nanoparticles decorated with plasma treated Ti3C2Tx was further confirmed by transient photocurrent response and time-resolved photoluminescence analyses.


 Please wait while we load your content...
Please wait while we load your content...