An in-plane Co9S8@MoS2 heterostructure for the hydrogen evolution reaction in alkaline media†
Abstract
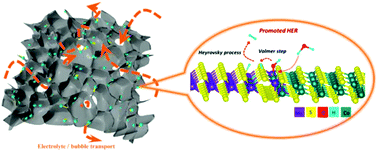
Transition metal sulfides have emerged as promising hydrogen evolution reaction (HER) electrocatalysts in acidic media due to high intrinsic activity. They exhibit inferior HER activity in alkaline media, however, owing to the sluggish water dissociation kinetics. Herein, in-plane MoS2/Co9S8 heterostructures are in situ grown on three-dimensional carbon network substrates with interconnected hierarchical pores by one-step pyrolysis to enhance the alkaline HER activity. The experiment results reveal that the HER kinetics of MoS2 is accelerated after the construction of heterostructures. The synthesized MoS2/Co9S8 heterostructures anchored on a three-dimensional interconnected hierarchical pore carbon network exhibit a lower overpotential of 177 mV than MoS2 (252 mV) at 10 mA cm−2 for the HER in 1 M KOH. The enhanced catalytic performance is mainly attributed to the accelerated water dissociation kinetics on the interface of MoS2 and Co9S8. In combination with DFT calculations, it is revealed that assembling the interface construction synergistically favors the chemisorption of protons and the cleavage of the O–H bonds of the H2O molecule, thus accelerating the kinetics of the HER. Moreover, the three-dimensional interconnected hierarchical pore carbon (3DC) network structure is beneficial for the circulation of the electrolyte and H2 spillover. This study demonstrates the present strategy as a facile route for fabricating efficient HER catalysts.


 Please wait while we load your content...
Please wait while we load your content...