Towards the surface hydroxyl species in CeO2 nanoparticles†
Abstract
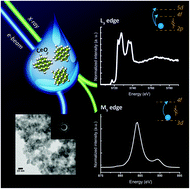
Understanding the complex chemistry of functional nanomaterials is of fundamental importance. Controlled synthesis and characterization at the atomic level is essential to gain deeper insight into the unique chemical reactivity exhibited by many nanomaterials. Cerium oxide nanoparticles have many industrial and commercial applications, resulting from very strong catalytic, pro- and anti-oxidant activity. However, the identity of the active species and the chemical mechanisms imparted by nanoceria remain elusive, impeding the further development of new applications. Here, we explore the behavior of cerium oxide nanoparticles of different sizes at different temperatures and trace the electronic structure changes by state-of-the-art soft and hard X-ray experiments combined with computational methods. We confirm the absence of the Ce(III) oxidation state at the surface of CeO2 nanoparticles, even for particles as small as 2 nm. Synchrotron X-ray absorption experiments at Ce L3 and M5 edges, combined with X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM) and small angle X-ray scattering (SAXS) and theoretical calculations demonstrate that in addition to the nanoceria charge stability, the formation of hydroxyl groups at the surface profoundly affects the chemical performance of these nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...