Dynamic visualization of the phase transformation path in LiFePO4 during delithiation†
Abstract
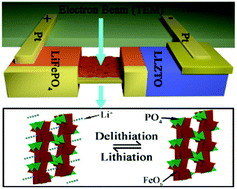
Rechargeable lithium-ion batteries have been widely used in portable electronic devices and electric vehicles over the last few decades. The electrochemical performance of lithium-ion batteries is mostly determined using electrode materials, which allow Li to insert/extract in their crystal structure. Conventionally, high-rate electrode materials store Li+via a solid-state reaction (i.e., the single-phase transformation path), and one exception is LiFePO4 (LFP). Although its two-phase transformation path has been widely demonstrated, the abnormal correlation between the lithiation/delithiation mechanism and the high rate performance of LFP is still controversial. Recently, the theory has suggested that the single-phase transformation path at a very low overpotential might be responsible for the abnormal phenomenon. However, direct observation of such a single-phase transformation has been rarely achieved, because once the overpotential is removed, the intermediate solid-solution phase LixFePO4 (0 < x < 1) should separate into thermodynamic LFP and FePO4 (FP). Here, the detailed delithiation path of LFP is directly observed using in situ transmission electron microscopy (TEM) based on a micro-sized solid-state battery (Pt/Li6.4La3Zr1.4Ta6O12/LFP). We first demonstrate a novel two-step solid-solution transformation path during the delithiation of LFP, showing direct evidence for the above assumption. These results provide a new insight into the solid-solution transformation mechanism of electrode materials.


 Please wait while we load your content...
Please wait while we load your content...