Bonding state synergy of the NiF2/Ni2P hybrid with the co-existence of covalent and ionic bonds and the application of this hybrid as a robust catalyst for the energy-relevant electrooxidation of water and urea†
Abstract
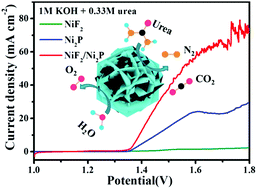
Among the energy-relevant electrochemical reactions, the electrochemical water and urea oxidation reactions are very significant for solving the increasing energy crisis and environmental pollution. Herein, the NiF2/Ni2P hybrid catalyst, in which covalent and ionic bonds co-existed, was found to be a very active catalyst for these electrochemical reactions occuring during the electrolysis of water. The bonding states of the covalent and ionic bonds were verified by the crystal structure and surface chemical state revealed by spectral analysis. As a bifunctional catalyst for the electrooxidation of water and urea, the NiF2/Ni2P hybrid structure demonstrated higher catalytic activity, kinetics and stability in the catalytic reaction than the individual components NiF2 and Ni2P under the same conditions. Specifically, an overpotential as low as 283 mV could drive the benchmark current density of 10 mA cm−2 for the oxygen evolution reaction, significantly lower than the overpotential required for the NiF2 (393 mV) and Ni2P materials (342 mV); the maximum current density for urea electrooxidation could reach 157.35 mA cm−2 at 1.53 V, which was much higher than those of NiF2 (23.55 mA cm−2) and Ni2P (102.72 mA cm−2). The catalytic performance also outperformed those of the recently reported similar advanced catalysts, and the high performance could be attributed to the highly exposed active sites, rough surface area, excellent charge transfer ability, and especially, the synergistic effects between the covalent and ionic bonds in the catalyst system. Using a commercial Pt/C catalyst as a cathode, the cell potential for urea-assisted water electrolysis could be reduced to 1.5 V to obtain the current density of nearly 40 mA cm−2 in a two-electrode system (Pt/C||NiF2/Ni2P), about 300 mV less than that required for water electrolysis in the general alkaline electrolyte. The current study demonstrates the significance of bonding state synergy in an advanced catalyst for water electrolysis and sheds some light on catalyst development in energy chemistry.


 Please wait while we load your content...
Please wait while we load your content...