Halide ion influence on the formation of nickel nanoparticles and their conversion into hollow nickel phosphide and sulphide nanocrystals†
Abstract
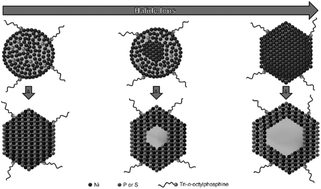
A dependence of the formation of tri-n-octylphosphine-capped Ni nanocrystals on the presence of halide ions during their synthesis is shown. For the application-oriented synthesis of Ni particles, this information can be crucial. Furthermore, Ni nanoparticles can be converted to nickel phosphide or sulphide by heating them up in the presence of a phosphorus or sulphur source, resulting in either solid or hollow nanocrystals, formed via the nanoscale Kirkendall effect, depending on the synthesis route. By adjusting the Ni crystallite size in the initial nanoparticles via the halide ion concentration the cavity size of the resulting hollow nanocrystals can be tuned, which is otherwise impossible to realise for particles of a similar total diameter by using this process. The synthesised hollow Ni3S2 nanocrystals exhibit a much sharper localised surface plasmon resonance (LSPR) band than all previously presented particles of this material, which is known to show molar extinction coefficients at the LSPR maximum similar to Au. This narrow linewidth could be explained by the nanoparticles’ high crystallinity resulting from the Kirkendall process and is interesting for various possible optical applications such as surface-enhanced Raman spectroscopy owing to the low cost of the involved materials compared to the widely used noble metals.


 Please wait while we load your content...
Please wait while we load your content...