Self-supported Co-doped FeNi carbonate hydroxide nanosheet array as a highly efficient electrocatalyst towards the oxygen evolution reaction in an alkaline solution†
Abstract
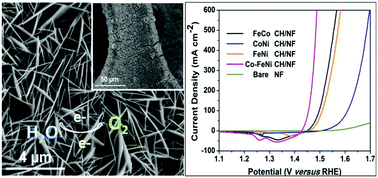
The evolution of molecular hydrogen from electrochemical water splitting has currently emerged as one of the promising strategies to address the ever-increasing energy crisis and environmental pollution. The development of low-cost, highly efficient and long-term durable electrocatalysts is still challenging for practical large-scale water splitting applications. Herein, a highly crystallized Co-doped FeNi carbonate hydroxide nanosheet array was strongly grown on a conductive nickel foam (Co-FeNi CH/NF) and was used as an oxygen evolution reaction (OER) electrocatalyst. The ternary Co-FeNi CH/NF electrode exhibited an improved OER activity and good durability for at least 20 hours. The electrode delivered current densities of 10 and 500 mA cm−2 at extremely low overpotentials of 202 and 254 mV along with a small Tafel slope of 37.5 mV dec−1 in a 1.0 M KOH electrolyte solution. The incorporation of an equivalent amount of cobalt into the trigonal FeNi CH crystal lattice significantly increased the electrochemical active surface area and reduced the electron transport resistance by effectively regulating the electronic structure of the resultant electrocatalyst. These interesting observations highlight the importance of the subtle combinations of the active earth-abundant metals with electronic structure modulations.


 Please wait while we load your content...
Please wait while we load your content...