Abstract
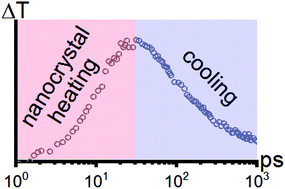
Ligand-to-nanocrystal heating and subsequent cooling to the environmental medium is investigated with infrared pump, electronic probe (IPEP) spectroscopy. Compared to solid films, solvated nanocrystals show faster ligand-to-nanocrystal heat equilibration (c. 11 ps versus c. 17 ps). Solvated nanocrystals also display more cooling of the hot ligand–nanocrystal complex on the experimentally measured time-scale, emphasizing the thermally insulating nature of semiconductor nanocrystal solids. Although heating transfer rates among solvents are all between 150 ps and 330 ps, cooling of the nanocrystal–ligand complex is slower, on average, in chlorinated solvents (c. 315 ps) compared to deuterated hydrocarbon solvents (c. 215 ps). Differences between chlorinated and hydrocarbon solvents show the importance of matching the vibrational energies of the solvent and the ligands for increasing the rate of heat transfer. Increases in the cooling time for poorer hydrocarbon solvents, in which nanocrystals aggregated, such as toluene, compared to better solvents, like methylcyclohexane, indicate that penetration of solvent into the ligand layer facilitates improved heat transfer to the matrix.


 Please wait while we load your content...
Please wait while we load your content...