Development of a hybrid nanocarrier-recognizing tumor vasculature and penetrating the BBB for glioblastoma multi-targeting therapy†
Abstract
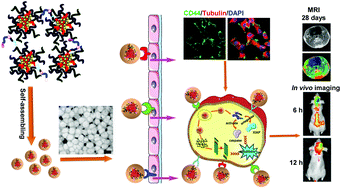
The success of glioma chemotherapy is hampered by poor drug penetration ability across the blood–brain barrier (BBB) and low intratumoral drug concentration. Novel tumor-targeted delivery systems are useful in specifically accumulating in the tumor foci and penetrating into the glioma core after entering into the brain. Here we show that a multi-targeting hybrid nanocarrier (Pep-MLHA HNPs) system based on hyaluronic acid (HA)-modified polymer and a functional peptide possesses multi-target capability and stronger penetration ability into the core of three-dimensional tumor spheroids, could migrate efficiently across the BBB in vitro. The intensity of the Pep-MLHA HNPs after transporting across the BBB was 5.2-fold and 5.6-fold higher than that of ML NPs in C6 and U87 cells, respectively. More interestingly, this multi-targeting hybrid system displayed high colloidal stability in PBS solution, and weak negative zeta potential (−1.99 ± 0.655 mV) minimizing nonspecific interactions with plasma proteins and promoting long-term circulation in vivo. Additionally, the multi-targeting hybrid system induced enhanced tumor localization in U87 in situ-bearing nude mice and xenograft-bearing nude mice after systemic administration. Furthermore, docetaxel (DTX)-loaded Pep-MLHA HNPs showed negligible systemic toxicity and enhanced therapeutic efficacy, with significantly improved survival rates in intracranial C6 glioma-bearing rats. The 50% survival rate of DTX/Pep-MLHA HNPs-treated rats (40 days) was significantly longer than that of rats treated with NS (22 days), Taxotere® (25 days), DTX/ML NPs (25 days), DTX/Pep NPs (32 days) and DTX/MLHA NPs (29 days). All the results suggested that the multi-targeting hybrid nanocarrier system is promising for glioma treatment.


 Please wait while we load your content...
Please wait while we load your content...