Investigating the luminescence mechanism of Mn-doped CsPb(Br/Cl)3 nanocrystals†
Abstract
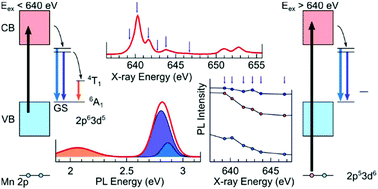
Inorganic lead halide perovskite CsPbX3 (X = Cl, Br, or I) nanocrystals are promising candidate materials for light-emitting devices and optoelectronics. Mn-Doped CsPbX3 is of particular interest, as the Mn-doping introduces an additional emission band, making this material a promising white-light emitter. In this study, Mn-doped CsPb(Br/Cl)3 nanocrystals are prepared at room-temperature and ambient pressure. The chemical environment of Mn, and the luminescence of these nanocrystals are analyzed in detail using X-ray diffraction (XRD), extended X-ray absorption fine structure (EXAFS), X-ray absorption near-edge structure (XANES) and X-ray excited optical luminescence (XEOL). Although the introduction of Mn does not alter the long-range order of the CsPbX3 crystal, it leads to a local lattice contraction with the bond length of Mn–X much shorter than Pb–X. We also find excitation energy-dependence in both the intensity and wavelength of the perovskite excitonic emission band, while only in intensity of the Mn emission band. Detailed fitting of the XEOL reveals that the perovskite emission band is dual-channel, and it is the excitation energy-dependent intensity variation of these two channels that drives the observed red-shift of the combined emission band. Our findings also confirm that the Mn emission band is driven by exciton-Mn energy transfer and clarify the Mn chemical environment and the luminescence mechanism in Mn-doped CsPb(Br/Cl)3 nanocrystals.


 Please wait while we load your content...
Please wait while we load your content...